September 14, 2020 – Ontario, Canada – SignPost Cancer Dx Inc. (“SignPost” or “company”), a company developing an advanced molecular diagnostic test to identify invasive breast cancer is pleased to be presenting at the Midwest Venture Capital Consortium. The event will be held virtually on Monday, September 14, 2020, from 3-4pmCT.
SignPost will be discussing the latest advances in diagnostic testing for breast cancer, upcoming corporate milestones, and the key investment highlights of the company.
“I am honored to have been selected to participate in the Midwest Venture Capital Consortium. Addressing women’s health, especially through early detection of breast cancer, can have a tremendous impact on the healthcare system and society at large,” stated Peter Blaney of SignPost. “Presenting in front of the Midwest Venture Capital Consortium provides SignPost the platform to share its investment opportunity with top-tier professionals who have an eye for early-stage companies.”
About the Midwest Venture Capital Consortium
The Midwest Venture Capital Consortium consists of 33 independent, primarily Midwest-based venture capital firms that have established a consortium to serve Midwest-based start-ups’ funding needs as well as investors seeking the Midwest’s demonstrated exceptional venture capital returns. The consortium, with a combined $2+ billion in cash available for investment, is expected to accelerate Midwest venture capital activity by establishing a virtual one-stop venture capital shop for aspiring entrepreneurs.
Such member firms include: Alumni Ventures Group, Boomerang Catapult, Cultivation Capital, Flyover Capital, Grand Ventures, Illinois Ventures, Loud Capital, Mutual Capital Partners, VCapital, and Wakestream Ventures while most of the Members remain confidential. Consortium members have people on the ground throughout the Midwest plus some representation on the coasts.
About SignPost Cancer Dx Inc.
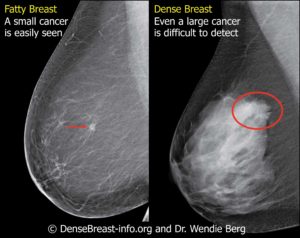
SignPost Cancer Dx Inc. (“SignPost”) is a company which has developed an advanced molecular diagnostic test, BreastDefense, to identify invasive breast cancer. BreastDefense is a lab-developed molecular test (LDT) that does not require FDA approval. SignPost is working with Dynacare, a wholly-owned subsidiary of LabCorp, to complete validation of BreastDefense. SignPost was founded and is managed by Induran Ventures, a Venture Philanthropy General Partnership which strives to achieve both outsized returns and massive social impact through their projects. For more information, please visit www.signpostcancerdx.com.
Forward Looking Statements
This press release includes forward-looking statements including, but not limited to, statements related to the development of our technology, our operations and business strategy, our expected financial results, and corporate updates. The forward-looking statements contained in this press release are based on management’s current expectations and are subject to substantial risks, uncertainty and changes in circumstances. Actual results may differ materially from those expressed by these expectations due to risks and uncertainties. Forward-looking statements speak only as of the date of this press release, and we undertake no obligation to review or update any forward-looking statement except as may be required by applicable law.
SignPost Cancer Dx Inc.:
Peter Blaney
Chief Executive Officer
SignPost Cancer Dx Inc.
Tel: +1 613.532.1290
Email: peterblaney@induranventures.com
Investor Contact:
Jennifer K. Zimmons, Ph.D.
Investor Relations
Zimmons International Communications
Tel: +1 917.214.3514
Email: jzimmons@zimmonsic.com
Source: SignPost Cancer Dx Inc.
###