START WITH CERTAINTY
 Why BreastDefense?
Why BreastDefense?
BreastDefense answers a very simple but fundamental question. Is this tumor an indolent lesion, or an invasive breast cancer? This is a question that no other test can reliably answer. For breast cancer, the most common screening method - mammography - can be inaccurate. The New England Journal of Medicine estimates 31% of all breast cancer cases may be misdiagnosed.
BreastDefense does not rely on images, it is a lab developed molecular test.
Thousands of women are being misdiagnosed.
This needs to change.
With a simple blood draw, BreastDefense aims to distinguish benign and malignant tissue with a test that balances 98.5% accuracy for both sensitivity and specificity.
The Harm of Over-Diagnosis
What many women don't realize is the potential harm of over diagnosis, not to be confused with false positives. It is when a mammogram detects non-invasive or benign cancer which is unlikely to cause a problem. Currently, it can be difficult to tell in the early stages, which can lead to unnecessary treatment.
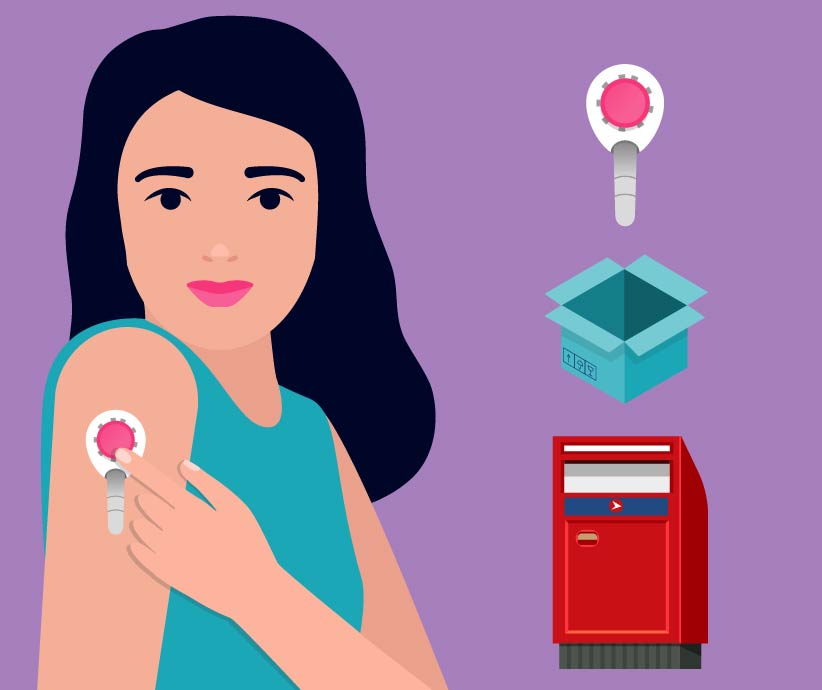
How it would work
Step 1 Request a self-collection blood kit.
Step 2 Client returns kit to SignPost lab for analysis.
Step 3 The blood results are sent to the client and her health practitioner.
Step 4 Client and health practitioner decide next steps.