|
Dear Friends,
As the holiday season begins, we hope that this update finds you and your family safe and healthy. With 2020 coming to a close, we wanted to take the time to review several positive milestones, advancements, and achievements as well as highlight new opportunities that are on the horizon for 2021.
The summary below reviews the highlights, turning points, and milestones of 2020.
1. Hired US Investor Relations Professional
To increase corporate visibility, SignPost engaged Zimmons International Communications, Inc. (ZIC). Jennifer K. Zimmons, PhD specializes in working with emerging companies and focuses on communications and corporate outreach to family offices, high net-worth investors, and boutique healthcare funds. Dr. Zimmons is also the Chair of the Program Committee for Women in Bio (New York). Over the summer and into the fall, ZIC has been involved with: arranging introductions to various types of family offices, boutique investors, and strategic parties; creating various investor materials and press releases; and identifying presentation opportunities for SignPost to highlight the company and its science.
i. Featured Speaker and Presenter at Women In Bio’s October Breast Cancer Awareness Panel
In October, SignPost was featured in Women In Bio’s (WIB) online event entitled “Females Pioneering Accurate Breast Cancer Detection and Innovative Ways of Healing”. WIB is an international group with over 10,000 members committed to highlighting females in the life sciences and in companies’ instrumental in supporting women’s health.
ii. Women of Influence and Strategic Introductions
As we round out the year, we remain focused on identifying women of influence in the investment and healthcare areas. Based on recent outreach efforts, we feel individuals with the aforementioned profile are ideally positioned to be supporters for BreastDefense and for investment and board positions.
2. Filed Provisional Patent
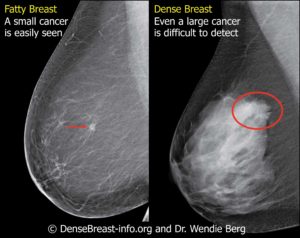
In July 2020, SignPost filed its provisional patent for the company’s lead diagnostic test – BreastDefense. The patent is for the DNA Methylation Multivariate Biomarkers offering 98.5% specificity and sensitivity (false positives and false negatives). Having the provisional patent in place is a very significant milestone for SignPost. The provisional patent allows SignPost to more openly share critical information with development partners, investors, and strategic entities.
3. The Cancer Screening Marketplace
The cancer screening marketplace saw some big deals this year.
Earlier in September, the biotech firm, Illumina, announced it will buy back cancer-detection start-up GRAIL, Inc. in an $8 billion deal. GRAIL is developing a blood-based cancer test called Galleri™, a medical test that can detect dozens of types of cancer. While GRAIL is developing a “pan-cancer diagnostic”, from published reports, we believe SignPost has developed a more accurate test specifically for breast cancer where our specificity and sensitivity is better than 98.5%.
In late October 2020, Exact Sciences (Nasdaq: EXAS) announced the intention to buy Thrive Earlier Detection, a private blood-based, multi-cancer screening company. The transaction was comprised of cash and stock consideration of up to $2.15 billion.
4. Why Companies Such as SignPost are Critical to Advancing Cancer Treatment Therapies
Dr. Azra Raza talks with Dr. Peter Attia about her new book The Cell in the following podcast. Take a listen as Dr. Raza discusses the content of her book which takes a critical look at survival rates of cancer for patients (a study of novel cancer treatment outcomes from 2000 to 2016 only increased survival by 2.4 months!) and why early detection is the way forward. It is a fascinating podcast. The key times discussing early detection are at 29min-31min and 1hr:24min -1hr:40min.
In closing, we want to thank you for your continued interest in SignPost. As you can see, there are many opportunities that we look forward to exploring in 2021. We wish the best in safety and health for you and your family during this holiday season.
Peter Blaney
Chief Executive Officer
SignPost Cancer Dx Inc.
Tel: +1 613.532.1290
E: peterblaney@induranventures.com
Investor and Press Contact:
Jennifer K. Zimmons, Ph.D.
Investor Relations
Zimmons International Communications
Tel: +1 917.214.3514
Email: jzimmons@zimmonsic.com
Source: SignPost Cancer Dx Inc.
### |